Toward Enhancing Clinical Trial Diversity
Robert A. Tumasian III
In research and development, the characteristics of a clinical trial population should match, or at least closely resemble, those of the population affected by the health condition that is meant to be targeted by the experimental treatment. However, some groups are often disproportionately included, and at times not captured at all, in clinical trials compared to the groups’ representation in the disease.
For example, this has been seen extensively in confirmatory Phase III trials assessing COVID-19 interventions, where the breakdown of the racial and ethnic backgrounds of enrolled subjects was not commensurate with the number of individuals from those populations who were at risk of infection. Lack of inclusion of racial and ethnic minority groups in clinical trials has persisted in the U.S. in almost every therapeutic area for some time now. From 2017–2020, the U.S. Food and Drug Administration (FDA) approved 206 novel drugs (i.e., compounds that offer a new way to treat specific medical ailments that are dissimilar to any previously approved FDA product). According to data from 181,387 participants, the aggregate of the trial populations was 69–77% White, 11–18% Hispanic, 7–11% Black, and 6–11% Asian each year, as shown in Table 1.
Some content is only viewable by ASA Members. Please login or become an ASA member to gain access.
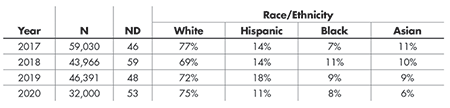
Table 1—Percentage of Subjects Enrolled in Successful Novel Drug Trials by Race and Ethnicity, 2017—2020, According to FDA Center for Drug Evaluation and Research (CDER) Drug Trials Snapshots
The percentages of all other races (American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, Other, and Unknown/Unreported) and ethnicities (Non-Hispanic and Unknown/Unreported) combined make up to 100% for both categories. The total number of trial participants and novel drugs approved each year is denoted by N and ND, respectively.
In 2021, most of the 50 novel drug trials leading to FDA approval also had incongruent representation of particular racial and ethnic groups. For instance, the population of the Phase III EMERGE trial, geared toward testing the effectiveness of Aduhelm in decelerating cognitive and functional impairment among individuals with early Alzheimer’s disease, was 78% White, 8% Asian, 4% Hispanic, and 0.7% Black. However, according to a 2020 report from the Alzheimer’s Association, older Black Americans are about twice as likely to have Alzheimer’s or other dementias as older whites, and older Hispanics are roughly 1.5 times as likely to have Alzheimer’s or other dementias as older whites.
Although the data presented here only reflects novel drug trials that yielded a statistically significant outcome in favor of the drug, it demonstrates the urgent need to enhance inclusivity in clinical trial populations. Doing so will enable more comprehensive safety and efficacy evaluation, maximize the generalizability of study results (that is, minimize extrapolation), and improve post-marketing surveillance.
This is a crucial task, because multiple groups can react very differently to certain drug products and alternative interventions; there are many disease subpopulations with known differences in morbidity and/or mortality.
Correlations between treatment response and race, ancestry, genetic variation, and ethnicity have also been described at length in the literature.
The gravity of this issue has prompted many agencies to take action, including the National Academies of Sciences, Engineering, and Medicine (NASEM); White House Office of Science and Technology Policy (OSTP); and FDA.
The FDA has been markedly proactive in helping to attenuate concerns about differential access to clinical studies by launching and promoting diversity-driven programs (the Enhance EQUITY Initiative) and adopting useful guidance documents to assist sponsors (biotech and pharmaceutical companies, contract research organizations, and universities) in enriching clinical trial populations.
For instance, in November 2020, the FDA released a constructive set of recommendations for mitigating both demographic (race, ethnicity, and age) and non-demographic (co-morbidity and disability) disparities in clinical trials, with numerous methods that can be employed to increase trial accessibility and inclusivity. Others are displayed in Figure 1, which contains numerous methods that can be employed to increase trial accessibility and inclusivity.
Figure 1. Interconnected dimensions of diversity (https://tinyurl.com/3h6xwhvh).
One technique is to broaden eligibility criteria. There are situations in which small sample sizes are inevitable, such as rare disease settings; exclusion is required due to concomitant illnesses or medications; or the risk outweighs the potential benefit (e.g., pregnant women sometimes should not be included in early studies due to unknown effects on the fetus or infant). However, loosening eligibility criteria in later phases of the drug assessment process can allow for better performance measurement in the population that is expected to use the drug and thus strengthen approval and labeling decisions.
If an inclusion criterion is not necessary to ensure the safety of subjects or accomplish the goals of the study, then its elimination should be strongly considered based on clinical judgment. It is also essential to thoroughly examine a drug’s safety profile in the initial stages of development to determine whether less-restrictive inclusion criteria can be used in subsequent confirmatory trials. Adaptive designs can also be implemented to address safety concerns in a smaller group of the population of interest, where the trial may begin with a certain subgroup and then be extended to a larger population according to a pre-specified interim analysis of the accrued safety data.
The concept of transportability has been shared in previous work as well, and insinuates that, under careful assumptions (that are often quite stringent and arduous to verify), treatment effects identified in clinical trials can be transferred to external populations that were not sufficiently included, but further exploration is needed to validate procedures in this landscape.
Another approach is to design the trial meticulously in a manner that tackles elements that may influence enrollment or retention, taking into account the characteristics of the target population. For example, time limitations may prevent some individuals from participating in a trial, but this could be avoided by lowering the frequency of study visits in the design stage.
Others may be constrained by different things, such as transportation costs or family responsibilities. These could be averted by choosing sites that are closest in distance to the target population or decentralizing the trial. In a decentralized trial, also known as home-based or site-less, subjects complete most of the study at home without having to repeatedly travel to a clinical site. One possible disadvantage is that this method often relies heavily on the integration of electronics and other technology, so it is critical to guarantee that the trial population will be able to operate any and all tools properly and without major difficulty. This could be problematic if trial participation is composed of older individuals.
An advantage is that decentralization can help give people in isolated or sparse geographic regions, such as rural areas, greater opportunities to participate in clinical studies. Moreover, engaging the population of interest in the preparation of the trial protocol can facilitate diversifying recruitment; working directly with members of the target population to identify specific factors that may deter participation can be instrumental in improving the trial both before and after commencement.
Ongoing public distrust in science has also hindered clinical trial diversity. Historically, some groups have been mistreated or exposed to unreasonable harm in clinical experiments (think of the Tuskegee study), and this has heightened the hesitancy of some populations to partake in health programs, including clinical studies. Unfortunately, this wariness toward science has been exacerbated by the spread of false and deceitful information about the COVID-19 pandemic. Expanding clinical trial populations requires fortifying faith in scientific practices.
In the clinical trial setting, this entails being clear and transparent at all stages of the study, particularly during the screening phase, when explaining each component of the study to prospective subjects who may not be familiar with the clinical trial process or have knowledge of biomedical principles. Screeners must ensure that each participant has a complete understanding of the trial and its risks, and that all of their questions are answered accurately and entirely, before obtaining informed consent.
Emphasis should also be placed on incorporating insights from the community into clinical research efforts. For instance, it has been shown that input from community clinicians, who typically have strong relationships with their patients, can be a key driver in an individual’s choice to participate in a trial; however, many of these clinicians have little experience in clinical trials or are unaware of study opportunities and thus cannot offer judicious advice or suggestions, which can leave their patients (especially those in vulnerable populations) feeling apprehensive.
Fostering confidence in scientific research involves collaboration between all stakeholders—industry, medical societies, academic institutions, patient groups, and site coordinators—to (1) educate the public and improve health literacy; (2) train study staff, physicians, and other medical professionals on clinical trial design and analysis, and in measures that can be taken to reduce barriers related to clinical trial participation (including demographic, environmental, lingual, logistical, religious, sociocultural, and socioeconomic factors); (3) publish results in journals or other resources that are free and available to everyone; and (4) devise strategies to convey findings in ways that can be absorbed by all audiences, regardless of their field(s) of expertise.
Last April, the FDA also unveiled a framework for preparing a clinical trial diversity plan that encourages investigators to (1) detail any previous indications of differential application or use of currently available treatments among racial and ethnic groups with the disease under study, or a comparable disease, that may have to be accounted for in the intended trial; (2) outline their goals and procedures for enrolling, retaining, and collecting data from underserved racial and ethnic participants, based in part on disease epidemiology and any existing methods used in past similar studies that were efficacious; and (3) explain how their analyses will adjust for different populations to gain results that are more encompassing and allow detecting heterogeneous effects in various subgroups.
Prioritizing increased representation of marginalized populations in clinical trials requires immediate government attention. As of now, all of the recommendations in the two FDA guidance documents discussed above are voluntary, although sponsors will soon be mandated to submit diversity plans per the recent passing of the 2023 Omnibus Appropriations Bills, which is a promising step forward.
Legislation is still being deliberated (e.g., the DEPICT Act) that will contribute to combating underrepresentation of racial and ethnic minorities in clinical trials by establishing statutes that would be binding on every sector.
Two additional options to contemplate are obligatory subgroup analyses and the sensibility and ethical aspect of federal incentives or reimbursements for trials that have populations with adequate inclusion of underserved groups. However, acquiring reliable data about the demographic and non-demographic summaries of some populations can be challenging, so creating an infrastructure that codifies and stores this information, based on evolving real-world evidence (e.g., patient records) and stratified by disease area, should be considered.
This issue is further complicated by discord about how to delineate race and ethnicity, which has been a primary objective of the White House Office of Management and Budget (OMB).
It is also important to mention that there are statistical implications associated with enhancing clinical trial diversity that must be addressed throughout a product’s lifecycle, such as how study outcomes are defined, analytical methods are selected, and results are interpreted. For example, assessing a drug in a wider population can yield data with higher variability, which must be balanced against the necessity to evaluate the target population or disease state correctly.
Altogether, it is paramount to develop and employ more-inclusive recruitment practices in clinical trials, to ensure high-quality safety and efficacy results that represent the population with the condition at hand who are anticipated to use the drug under investigation.
Simply increasing samples sizes to improve inclusivity usually is not feasible. Trial opportunities must also be advertised better.
Finally, determining how to lessen the burden on not only patients but also study personnel, and diversifying all levels of the biomedical research enterprise, will greatly help curb this long-standing problem. Figure 2 briefly recapitulates some imperative matters that must be rectified.
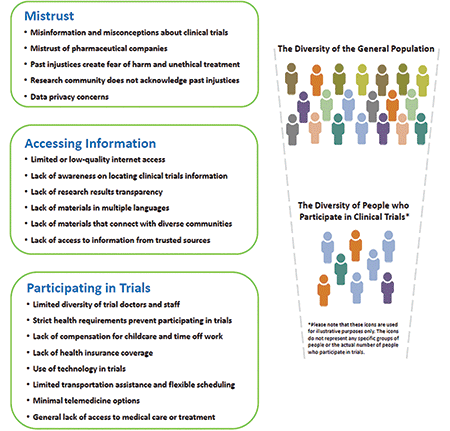
Figure 2. Barriers preventing diversity in clinical trial populations (https://tinyurl.com/mr3w84jv).
Further Reading
Lolic, Milena, et al. 2021. U.S. racial and ethnic participation in global clinical trials by therapeutic areas. Journal of Clinical Pharmacy and Therapeutics 46.6:1576–1581.
Moore, Kenneth T., et al. 2022. The Importance of Diversity and Inclusion in Drug Development and Clinical Trial Conduct. Journal of Clinical Pharmacology 62.12:1475–1479.
National Academies of Sciences, Engineering, and Medicine. 2022. Improving representation in clinical trials and research: building research equity for women and underrepresented groups.
Sharma, Ashwarya, and Latha Palaniappan. 2021. Improving diversity in medical research. Nature Reviews Disease Primers 7.1:1–2.
Woods-Burnham, Leanne, et al. 2021. The role of diverse populations in U.S. clinical trials. Med 2.1:21–24.
About the Author
Robert A. Tumasian III is a PhD candidate in biostatistics at Columbia University. His work focuses broadly on optimizing clinical trial designs and analyses to accelerate the drug evaluation process and expedite the delivery of safe and effective treatments to the community.










